
Lasting Love Deserves Lasting Itch Relief



Cytopoint® provides long-lasting itch relief for dogs with allergic dermatitis or atopic dermatitis. Ask your veterinarian how Cytopoint can provide lasting relief for your itchy dog, so everyone can get back to enjoying life’s precious moments.
Stay On Top of Your Dog’s Allergic Itch Treatments With Worry-Free Reminders

Cytopoint works like your dog’s own immune system. It is specifically designed to target and neutralize one of the main proteins that send itch signals to your dog’s brain, which triggers scratching, licking and chewing.
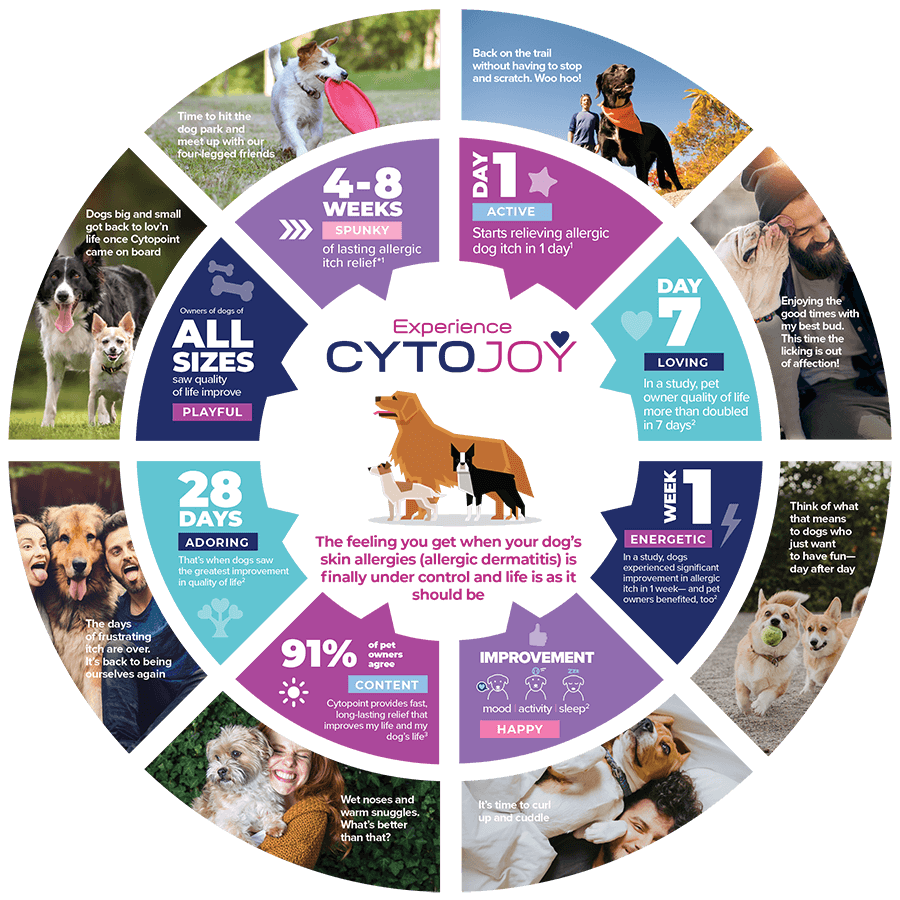
CytoJoy: the feeling you get when allergic dog itch is finally under control and life is as it should be.
Activate reminders and you’ll automatically receive appointment reminders by email or text.

See how Cytopoint helped dogs with allergic itch get back to enjoying life again!
See if your dog needs allergic itch treatment, learn about Cytopoint, track treatment progress, and set appointment reminders.
Indications: Cytopoint has been shown to be effective for the treatment of dogs against allergic dermatitis and atopic dermatitis.
*Repeat administration every 4 to 8 weeks as needed in the individual patient.2
References: